|
The preclinical profiling is a risk assessment that uses in vitro and in vivo properties of small molecules to estimate safety. In general, drug candidates are evaluated in respect to the absorption, distribution, metabolism, excretion, and toxicity (ADMET). Current preclinical assays often lack throughput and efficiency. In addition, there are limited assays available to estimate critical pharmacokinetic variables such as clearance and bioavailability. The research goal of the Arnold Group is to increase the throughput of existing preclinical assays and developed new methods to improve in vivo safety. |
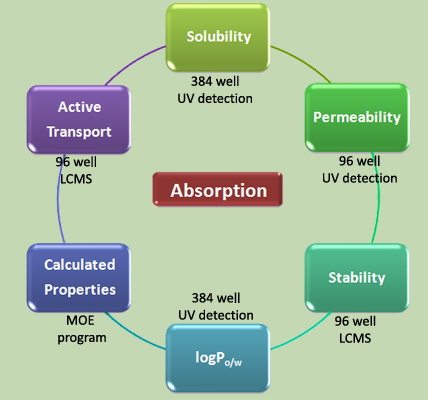
The in vivo absorption can be estimated with diverse in vitro preclinical assays probing different compound properties. Most of these assays can be carried out in high throughput. Important for us is the integration of algorithms that enable the calculation of compound properties in respect to absorption. In our group, we are using MOE (molecular operation environment) to generate compound descriptors and multi-well plate-based assays to determine compound properties in respect to solubility, permeability, stability, logP (octanol/water), and active cellular transport. |
 |
 |
The binding to plasma proteins (albumins and globulins) and tissue proteins influences the distribution of drugs molecules in the body. Plasma protein binding is also influencing the metabolism and clearance of drugs. Our group has developed a fluorescence-based competition assay that determines plasma protein binding in high throughput and in a dose-dependent manner. We are also working on an assay to determine drug–tissue and drug–tissue protein binding. |
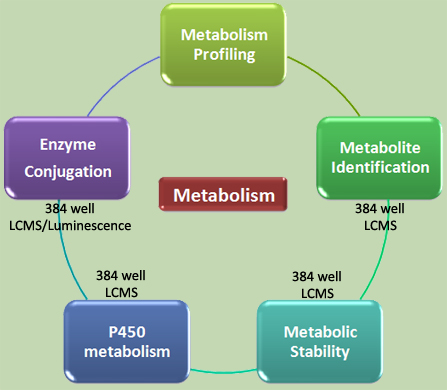
The metabolic pathway of a drug can be probed with many in vitro biochemical and cell-based assays. The conversion of drug candidates in the presence of liver microsomes or hepatocytes can be qualified and quantified by LCMS/MS. A more detailed characterization of metabolic products can be achieved by using isolated metabolic enzymes such as the P450 family, UDP glucuronosyltransferases, glycosyltransferase, sulfotransferases, or glutatione-S-transferases. In addition, the application of kinetic experiments with fluorescent enzyme substrates enables a quick identification of competitive enzyme inhibitors, thus substrates.
|
|
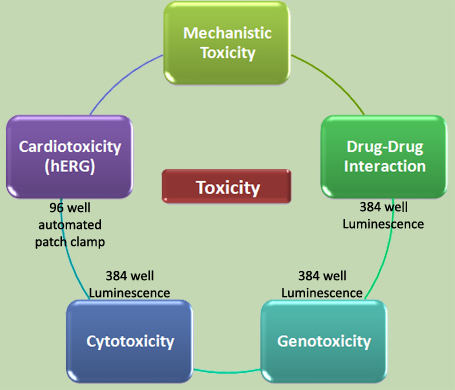 |
Cardiotoxicity, hepatotoxicity, and neurotoxicity continue to be the most common underlying issues for drug attrition at all stages of development. In a HTS format, cells like hepatocytes for liver toxicity, renal proximal epithelial cells for nephrotoxicity, vascular endothelial cells for vascular toxicity, neuronal or glial cells for neurotoxicity, and cardiomyocytes for cardiotoxicity are used. Cytotoxicity endpoints such as membrane integrity, cellular metabolite content, mitochondrial functions, lysosomal functions, and apoptosis are used for the screening of organ-specific toxicity as well. Genotoxicity (gene mutation and chromosomal aberrations) is determined by forward mutation assays (thymine kinase or hypoxanthine-guanine phosporibosyl transferase). Cardiotoxicity is analyzed with HEK293T cells transfected with the human ether-a-go-go related gene (hERG). We determine the effect of drug candidates on the influx of potassium ions using automated patch clamp. |
Investigation of compounds that might have unwanted CNS activity such as our new drug candidates for asthma are investigated in vivo using the rotarod experiment. Therefore, we are training Swiss Webster mice to stay on a rotating rod for a certain amount of time. All students in the Arnold group learn how to handle animals and carry out these studies under very strict IACUC protocol guidelines. |
 |
 |
Pharmacokinetic profiling is one of the most important study in drug discovery. We are using mice and rats under our certified IACUC protocol to investigate lead compounds that have distinct pharmacological activity. Using different routes of administration, we quantify the amount of compound in the blood and different organs such as the lung for our asthma research. The amount of compound at different time points are quantified by LCMS/MS in the multiple reaction monitoring (MRM) mode. Our Shimadzu 8040 instrument equipped with online solid-phase extraction has the necessary speed and sensitivity to support these sophisticated studies. |
Essential for our asthma program is the measurement of airway hyperresponsiveness, a method that allows us to determine how airway restriction can be elevated by our new drug candidates. We are using a none-evasive instrument from DSI Buxco®FinePoint, which allows for the independent measurement of nasal and thoracic flows. The airway resistance is related to the phase difference between these two flows and is a direct measurement of airway resistance. |
|